

 Secure and encrypted payment processing
Secure and encrypted payment processing We ship to over 40 countries including the USA, UK, Europe, Australia and Japan
We ship to over 40 countries including the USA, UK, Europe, Australia and Japan Guaranteed refund or reship if you haven't received your order
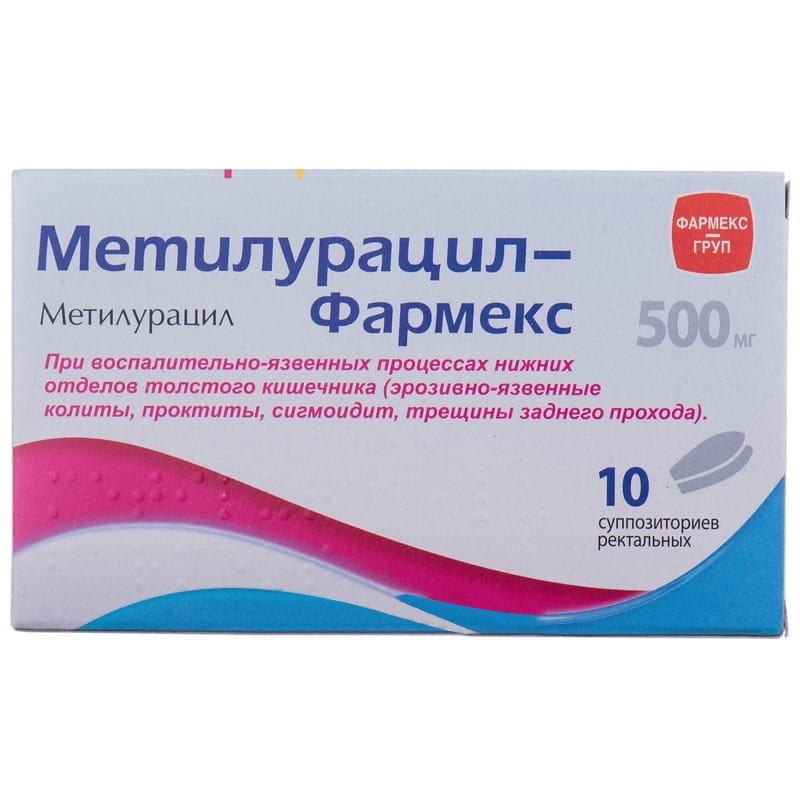
Guaranteed refund or reship if you haven't received your orderActive substance: methyluracilum;
1 suppository contains methyluracil - 500 mg;
excipient: solid fat.
Rectal suppositories.
Basic physical and chemical properties: smooth suppositories of white or almost white color with a uniform consistency. The presence of a funnel-shaped recess and an air rod are allowed.
Non-steroidal anabolic agent. code akh a14v.
Pharmacodynamics
The drug has anabolic and anti-catabolic properties. It accelerates the regeneration processes, wound healing, stimulates cellular and humoral immunity, has an anti-inflammatory effect. A specific property of the drug is a stimulating effect on erythro- and especially on leukopoiesis.
Pharmacokinetics
With the introduction of a suppository into the rectum, the drug is well and almost completely absorbed through the mucous membrane and appears in the blood after 20-30 minutes. The maximum concentration in the blood is reached after 1-2 hours. It is excreted mainly by the kidneys in the form of metabolites and conjugates and partially with feces unchanged.
Methyluracil is used in inflammatory and ulcerative processes of the lower parts of the large intestine (erosive-ulcerative colitis, proctitis, sigmoiditis, anal fissures).
Individual hypersensitivity to the components of the drug. in acute and chronic leukemic forms of leukemia (especially myeloid), lymphagranulomatosis, malignant bone marrow diseases.
Methyluracil enhances the effect of strophanthin, increases the effect of antibiotics and sulfa drugs.
The drug should be prescribed for mild forms of leukopenia. in case of moderate disease, the drug should be taken only after the resumption of impaired blood cell regeneration. in severe forms of hematopoiesis, do not prescribe methyluracil.
The drug should not be used during pregnancy and / or breastfeeding.
Until the patients individual reaction to the drug is clarified, one should refrain from driving vehicles or working with other mechanisms, given that during the treatment with methyluracil, adverse reactions from the nervous system such as dizziness are possible.
Before use, the suppository should be removed from the blister.
The drug is intended for rectal use. Adults and children over 14 years: 1 suppository 1–4 times a day. The course of treatment is from 7 days to 4 months, depending on the nature of the disease.
The drug should not be used to treat children under 14 years of age.
Overdose and toxic effects when using the drug have not been identified.
From the nervous system: headache, dizziness.
On the part of the skin and subcutaneous tissue: allergic reactions, including hyperemia, itching, skin rashes, urticaria.
Changes in the injection site: when the suppository is introduced into the rectum, a short burning sensation, itching is sometimes felt.
3 years.
Store in the original packaging at a temperature not exceeding 25 °С.
Keep out of the reach of children.
5 suppositories in a strip, 2 strips in a pack of cardboard.
Over the counter.
LLC “farmex group”.
Ukraine, 08301, Kiev region, g.Borispol, st. Shevchenko, building 100.
All cases of adverse reactions must be reported to the manufacturer:
Farmeks Group LLC, Ukraine, 08301, Kiev region, Boryspil, st. Shevchenko, building 100, tel .: +38 (044) 391–19–19, fax: +38 (044) 391–19–18 or through the form on the website: http://www.pharmex.com.ua/farmakonadzor