

 Secure and encrypted payment processing
Secure and encrypted payment processing We ship to over 40 countries including the USA, UK, Europe, Australia and Japan
We ship to over 40 countries including the USA, UK, Europe, Australia and Japan Guaranteed refund or reship if you haven't received your order
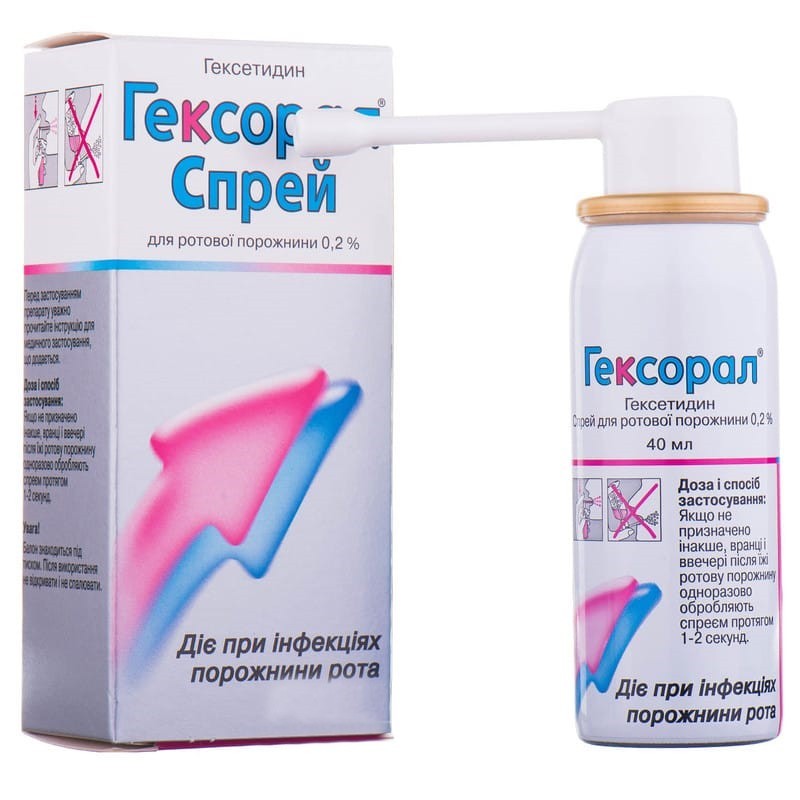
Guaranteed refund or reship if you haven't received your orderHexetidine is an active substance with a quick and lasting effect, which belongs to the group of local antiseptics used gingivodentno and oropharyngeal. It has antibacterial and antifungal effects against a wide range of pathogenic microorganisms that cause oropharyngeal infections. when applied, it also exhibits wound healing, hemostatic and local analgesic properties in the oral cavity and pharynx. hexetidine is a broad-spectrum antimicrobial agent. this agent is effective both in vivo and in vitro against gram-positive and gram-negative bacteria, as well as yeast (candida albicans) and fungi.
Specific pharmacokinetic studies on the use of Hexoral spray in humans have not been carried out.
When using the drug, plaque and residual concentrations of hexetidine on the mucous membranes can be determined. Studies using labeled hexetidine have shown that it can be detected on the tissues of the oral cavity from 8 to 10 hours after a single application, and in some cases, manifested on the tissues of the oral cavity 65 hours after treatment.
Pharmacokinetics There are no clinical data on the pharmacokinetics of hexetidine in humans.
The drug acts locally on the mucous membrane of the mouth, so it is expected that it is absorbed only in a small amount.
The high affinity of hexetidine with proteins and polymers with electronegative sites explains its binding to bacteria and directly contributes to its effect on the oral mucosa due to the residual concentration of hexetidine. This affinity also explains the binding of plaque and, therefore, the anti-plaque effect. The antibacterial effect can be determined 10-14 hours after application.
Absorption study after topical application of Hexoral® Spray or hexetidine in humans has not been performed.
Specific Research Hexoral Spray® or hexetidine in patients with impaired liver and / or kidney function and elderly patients was not performed.
Hexoral® is used for minor infections of the oral cavity, including candidiasis; as an adjuvant for the prevention and treatment of gingivitis; with sore throat and recurrent aphthous ulcers; to eliminate bad breath; before and after surgical interventions in dentistry.
Adults and children over 6 years old
Hexoral® spray is a tool for topical use in the oral cavity.
Technique of the procedure:
Apply no more than 3 times a day (depending on the severity of the disease or doctor’s recommendations).
The duration of treatment is determined by the doctor individually, depending on the severity and characteristics of the course of the disease.
Not used for treatment of symptoms that manifest over a long period.
Do not breathe, may cause laryngospasm.
You should consult your doctor before using the drug.
Hexoral® Spray is contraindicated in:
On the part of the immune system: hypersensitivity reactions, including urticaria, angioedema, allergic reactions, including laryngospasm, bronchospasm.
From the side of the nervous system: ageusia, dysgeusia, change in taste during 48 hours (the feeling of "sweet" can be changed twice to the feeling of "bitter").
From the respiratory system, chest and mediastinal organs: cough, shortness of breath, laryngospasm.
From the digestive tract: dry mouth, dysphagia, enlarged salivary glands, pain when swallowing. If the drug is accidentally swallowed, gastrointestinal disturbances may occur, especially nausea and vomiting.
On the part of the skin and subcutaneous tissue: allergic contact dermatitis, angioedema.
General disorders and condition at the place of use: local reactions, including reversible changes in the color of teeth and tongue; sensitivity of the mucous membrane, namely, burning, numbness; irritation (soreness, sensation of heat, itching) of the tongue and / or mucous membrane of the oral cavity; decreased sensitivity; paresthesia of the mucous membrane; inflammation vesicles; the appearance of ulcers on the mucous membrane.
The drug should be used with caution in patients with epilepsy. the drug can reduce the epileptic threshold and cause seizures in children. with caution, the drug should be used in patients with allergic reactions, including BA, especially in patients with allergies to acetylsalicylic acid.
The drug contains ethanol, so it should be used with caution in patients with liver diseases.
During the use of the drug should not be inhaled, as the spray can get into the respiratory tract and cause laryngospasm.
There is a risk of laryngospasm in children due to the presence of levomenthol in the composition of the drug.
With increased inflammation, treatment should be discontinued.
Long-term use is not recommended (do not use longer than 10 days without a doctor’s recommendation).
Use during pregnancy and lactation. Appropriate and well-controlled clinical studies of the use of hexetidine during pregnancy and lactation have not been conducted. It is not known whether hexetidine or its metabolites are excreted in breast milk. A small amount of hexetidine is absorbed systemically. It is unlikely that the use of hexetidine during pregnancy and lactation causes a risk to the fetus or child. However, hexetidine should not be used during pregnancy and lactation, unless the potential benefits of treatment for the mother outweigh the possible risks to the development of the fetus or child.
Children. Do not use Hexoral® spray in children under 6 years old.
The ability to influence the reaction rate when driving vehicles or other mechanisms. The drug contains ethanol. Drivers are not recommended to drive within 30 minutes after using the drug.
Interactions with other antiseptic drugs may occur. hexetidine can be inactivated with alkaline solutions.
Ingestion of a large amount of spray can cause nausea, so significant systemic absorption will not occur.
Due to the absorption of a sufficient amount of Hexoral spray® alcohol intoxication may occur due to the ethyl alcohol content. The concentration of hexetidine, which is contained in hexoral® spray for the oral cavity, non-toxic if the drug is used as directed. There are no cases of excessive use of hexetidine, which leads to hypersensitivity reactions. Keep out of the reach of children. In case of overdose, seek medical attention immediately.
At a temperature not exceeding 30 ° C to protect from light in the original packaging, out of the reach of children. expiration date - 3 years.
It is not recommended to use 6 months after opening.