

 Secure and encrypted payment processing
Secure and encrypted payment processing We ship to over 40 countries including the USA, UK, Europe, Australia and Japan
We ship to over 40 countries including the USA, UK, Europe, Australia and Japan Guaranteed refund or reship if you haven't received your order
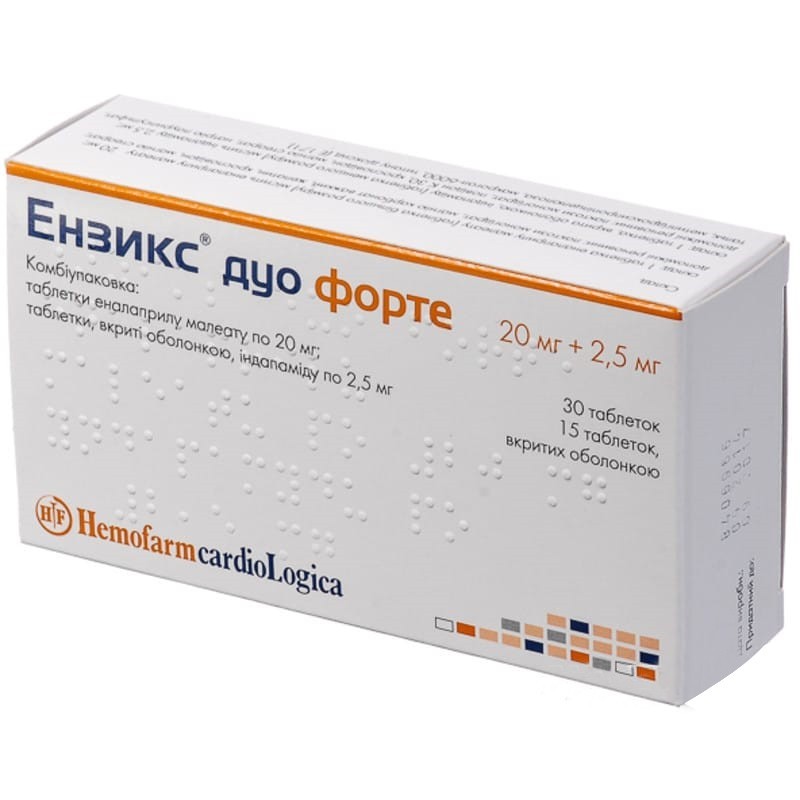
Guaranteed refund or reship if you haven't received your orderEnixix Duo Forte contains two separate drugs in one package: an apf enalapril inhibitor and an indapamide diuretic.
Enalapril. Enalapril maleate is an enalapril salt of maleic acid, a derivative of two amino acids - L-alanine and L-proline. ACE is a peptidyl peptidase, which acts as a catalyst for the conversion of angiotensin I into a substance that increases blood pressure, angiotensin II. After absorption, enalapril is hydrolyzed to form enalaprilat, which serves as an ACE inhibitor. The slowdown of ACE leads to a decrease in the concentration of angiotensin II in blood plasma, which leads, in turn, to a decrease in the activity of renin in blood plasma (due to the elimination of negative feedback with the release of renin) and to a decrease in the secretion of aldosterone.
ACE is identical to kinase II. Thus, enalapril can also block a decrease in the concentration of bradykinin, a potent peptide vasodepressor. Despite this, the role that this circumstance can play in the therapeutic effect of enalapril remains open to study.
Since the mechanism by which enalapril lowers blood pressure is considered to be the primary suppression of the renin-angiotensin-aldosterone system, enalapril is an antihypertensive drug even in the treatment of hypertensive patients with low renin levels.
The use of enalapril in patients with hypertension leads to a decrease in both indicators - blood pressure in both the recumbent and standing position, without increasing the heart rate.
Symptomatic translational or orthostatic hypotension is rare. In some patients, the development of an optimal decrease in blood pressure may require several weeks of therapy. The sudden cessation of enalapril is not associated with a rapid increase in blood pressure.
An effective inhibitory effect on ACE activity usually occurs 2–4 hours after oral administration of an individual dose of enalapril. The manifestation of antihypertensive activity can usually be seen after 1 hour, and the maximum decrease in blood pressure is achieved 4-6 hours after taking the drug. The duration of this effect is dose dependent. Despite this, if we proceed from the conditions of an overdose of the drug, then its antihypertensive and hemodynamic effects continue, as revealed, on average for at least 24 hours.
In experimental studies of hemodynamic effects involving patients with significantly increased blood pressure, a decrease in blood pressure was accompanied by a decrease in resistance in the peripheral arteries, an increase in cardiac blood flow with minor changes or even in the absence of any changes in heart rate. After taking enalapril, an increase in blood flow through the kidneys was observed; the glomerular filtration rate remained unchanged. There were no signs of sodium or water retention. Despite this, in patients who had a low glomerular filtration rate before treatment, this rate usually increased.
In the course of short-term clinical studies of patients with and without diabetes, as well as with kidney diseases, after enalapril administration, a decrease in the amount of protein in the urine and the release of IgG immunoglobulin in the urine could be observed.
If enalapril is taken simultaneously with diuretics such as thiazides, then its effect on lowering blood pressure is less additive. Enalapril can reduce or even prevent the development of hypokalemia due to the use of thiazides.
There is only limited experience with the use of this drug in pediatric patients with hypertension, that is, at the age of 6 years.
Indapamide. Indapamide is a non-thiazide sulfamide with an indole ring, which belongs to the family of diuretic drugs.At a dose of 2.5 mg / day, indapamide exhibits prolonged antihypertensive activity in humans. Experimental studies of dose dependence have shown that at a dose of 2.5 mg / day, the antihypertensive effect is maximum, and the diuretic effect is in preclinical limits.
At this antihypertensive dose of 2.5 mg / day, indapamide weakens the increased vascular response to norepinephrine in patients with high blood pressure and reduces OPSS and arteriolar resistance.
The involvement of the extrarenal, that is, extrarenal mechanism of action, in the antihypertensive effect has been demonstrated by maintaining its antihypertensive efficacy in patients with hypertension with functional absence of the kidneys.
The vascular mechanism of action of indapamide includes the following:
It has also been demonstrated that as a result of short, medium and long-term action in the case of patients with hypertension indapamide:
Finally, the simultaneous use of indapamide with other antihypertensive drugs (β-adrenergic receptor blockers, calcium channel blockers, angiotensin I-converting enzyme inhibitors) leads to improved hypertension control with an increased percentage of responders compared to what is observed in the case of monotherapy.
Pharmacokinetics
Enalapril. Absorption. After oral administration, enalapril is rapidly absorbed, Cmax enalapril in the blood plasma is detected within 1 hour. Based on the results of the identification of the drug in the urine, we can say that the degree of absorption of enalapril after oral administration of the enalapril tablet is approximately 60%. The presence of food in the gastrointestinal tract does not affect the oral administration of enalapril. After absorption, the oral dose of enalapril is rapidly and extensively hydrolyzed to form enalaprilat, a potent inhibitor of angiotensin I-converting enzyme. Cmax plasma enalaprilat is achieved 4 hours after ingestion. Effective Duration T½ taking into account the accumulation of enalaprilat after repeated administration of enalapril tablets, it is 11 hours. In individuals with normal renal function, equilibrium plasma concentrations of enalaprilat were reached after 4 days of treatment.
Distribution. In the whole range of concentrations that are characterized as therapeutically important, the binding of enalaprilat to plasma proteins should not exceed 60%.
Biotransformation. With the exception of conversion to enalaprilat formation, no noticeable signs of enalapril metabolism were observed.
The elimination of enalaprilat from the body occurs first by the renal route. The main components in the urine were enalaprilat, which was ≈40% of the dose, and unreformed enalapril (≈20%).
Renal failure. Excretion of enalapril and enalaprilat is increased in patients with renal failure. In patients with mild or moderate renal failure (creatinine clearance of 40-60 ml / min), the equilibrium concentration of enalaprilate (by AUC) was approximately 2 times higher than in patients with normal renal function after taking 5 mg 1 time per day .In case of serious impaired renal function (creatinine clearance of 30 ml / min), AUC increased by ≈8 times. Effective Duration T½ Enalaprilat after repeated administration of tablets was prolonged at this level of renal failure, and the time to reach equilibrium concentrations was delayed. Enalaprilat can be removed from the body using general hemodialysis of the circulatory system. Dialysis clearance is 62 ml / min.
Children and adults. Pharmacokinetic studies of the effect of multiple doses were conducted with the participation of 40 pediatric patients of male and female gender with hypertension from 2 months to ≤16 years of age. The studies concerned the determination of appropriate characteristics after daily oral administration of enalapril maleate at a dose of 0.07 to 0.14 mg / kg body weight. In this case, no significant differences were observed between the pharmacokinetic parameters in children and the corresponding characteristics for adults. The data obtained indicate that the increase in AUC (reduced to a normal dose rate per 1 kg of body weight per day) increases with age; nevertheless, no AUC growth was observed when the data were given in units of body area. In the case of equilibrium concentrations, the average effective T½ Enalaprilat accumulation was 14 hours.
Indapamide
Absorption. Indapamide is rapidly and completely absorbed in the digestive tract. Cmax in blood plasma is achieved 1–2 hours after administration. The stage of stable concentration occurs after 7 days of regular intake.
Distribution. Linking blood plasma proteins - 79%. T½ is 14-24 hours (on average - 18 hours).
The drug is metabolized in the liver and excreted in the form of inactive metabolites mainly by the kidneys (70% of the dose) and excreted in the feces (22%).
Patients at increased risk. Pharmacokinetic parameters do not change in patients with renal failure. In the presence of liver failure, the use of thiazide and thiazide-like diuretics can cause hepatic encephalopathy.
Ag
Applied in the case when monopreparations are ineffective.
1 tablet of enalapril maleate (20 mg) and 1 tablet of indapamide (2.5 mg) are taken orally in the morning at the same time. Depending on the dynamics of blood pressure indicators, the dose of enalapril maleate can be increased to twice a day (2 tablets of 20 mg).
The maximum daily dose of enalapril maleate is 40 mg (2 tablets of 20 mg), indapamide - 2.5 mg (1 tablet).
In chronic renal failure, the accumulation of enalapril occurs with a decrease in filtration of 10 ml / min. With creatinine clearance of 30–80 ml / min, the dose of enalapril maleate should be 10 mg / day (½ tablets of 20 mg).
Enalapril. hypersensitivity to enalapril and other APF inhibitors; the presence in the history of angioedema of idiopathic, hereditary, associated with treatment with APF inhibitors; impaired renal function (creatinine clearance 30 ml / min); During pregnancy and breastfeeding; age up to 18 years (efficacy and safety not established), porphyria.
Indapamide. Hypersensitivity to indapamide, other derivatives of sulfonamides or other components of the drug; anuria hypokalemia, severe hepatic (including encephalopathy) and / or renal failure; acute cerebrovascular accident; simultaneous use with drugs that extend the Q – T interval, pregnancy and lactation, age up to 18 years (efficacy and safety have not been established).
Undesirable side effects of the drugs are given below and are located in accordance with the class and frequency of manifestations. the frequency of manifestations of these effects is defined as follows: very often (1/10), often (1/100, 1/10), infrequently (1/1000 and 1/100), rarely (1/10 000 and 1/1000) and very rarely (1/10 000), including single messages.
Enalapril
Violation of the functions of the hematopoietic system and lymphatic system: infrequently - anemia (including aplastic and hemolytic); rarely - neutropenia, decreased hemoglobin concentration and hematocrit, thrombocytopenia, agranulocytosis, bone marrow depression, pancytopenia, lymphadenopathy, autoimmune diseases.
Metabolic dysfunction: infrequently - hypoglycemia.
Disorders of the functions of the nervous system and mental disorders: often - headache, depression; infrequently - confusion, drowsiness, insomnia and other sleep disorders, nervousness, paresthesia, dizziness and loss of consciousness, cramps, redness of the face, tinnitus (tinitis), discomfort and anxiety, high body temperature (fever); rarely - sleep anomalies, sleep disorders.
Vision disorders: very often - blurred vision.
Cardiovascular disease: very often - dizziness; often - low blood pressure (including with orthostatic hypotension), loss of consciousness, chest pain, heart rhythm disturbance, angina pectoris, tachycardia; infrequently - orthostatic hypotension, tachycardia, myocardial infarction or acute cerebrovascular accident - stroke, secondary manifestations of excessive hypotension, thromboembolism of the branches of the pulmonary artery are possible; rarely - Raynauds syndrome.
Respiratory diseases, thoracic and mediastinal disorders: very often - cough; often - shortness of breath (shortness of breath); infrequently - pharyngitis and tonsillitis, hoarseness, bronchospasm / asthma; dry cough; rarely, pulmonary infiltrate, rhinitis, allergic alveolitis / eosinophilic pneumonia.
Endocrine disorders of the secretion of antidiuretic hormone
Gastrointestinal diseases: very often - nausea; often - diarrhea, pain in the lower abdomen, a change in taste; infrequently - intestinal obstruction (ileus), pancreatitis, vomiting, dyspepsia, coprostasis, anorexia, gastritis, dry mouth, stomach ulcer and duodenal ulcer; rarely - stomatitis / aphthous ulcers, inflammation of the tongue (glossitis); very rarely - angioedema of the intestine.
Diseases of the liver and biliary tract: rarely - impaired liver function, hepatitis - hepatic cell (hepatocellular) or cholestatic, including with necrosis of the liver, stagnation of bile, cholestasis, including jaundice, liver failure.
Diseases of the skin and subcutaneous tissues: often - there have been reports of rashes, increased sensitivity of the skin / angioedema of the face, limbs, lips, tongue, glottis and / or larynx; infrequently - excessive sweating (hyperhidrosis), itchy skin (itching), urticaria, alopecia; rarely - polymorphic erythema, Stevens-Johnson syndrome, exfoliative dermatitis, toxic epidermal necrolysis, ordinary pemphigus, erythroderma.
The complex of symptoms reported may include some or all of the following symptoms: high body temperature (fever), inflammation of the serous membranes (serositis), vasculitis, myalgia and myositis, arthralgia and arthritis, a positive reaction to antinuclear antibodies (ANA) , increased ESR (ESR), eosinophilia, leukocytosis. Rashes, increased photosensitivity or other dermatological manifestations are also possible.
Diseases of the kidneys and urinary tract: very often - nausea; rarely - impaired renal function, renal failure, proteinuria; rarely - oliguria.
Diseases of the reproductive system and mammary glands: infrequently - impotence; rarely - gynecomastia.
General diseases and situational conditions for the administration of drugs: very often - asthenia; often fatigue.
Laboratory studies: often - hyperkalemia, increased plasma creatinine concentration; rarely - increased concentration of urea in the blood, hyponatremia; rarely - increased levels of liver enzymes, plasma bilirubin concentration.
Indapamide
Usually, drug treatment is well tolerated. Most clinical and laboratory adverse reactions are dose dependent and can be significantly reduced by applying the minimum effective dose.
Sometimes there are changes in the water-electrolyte balance:
Rarely:
Clinical manifestations
From the digestive tract: nausea, vomiting, constipation, dry mouth, very rarely - pancreatitis. In patients with liver failure, hepatic encephalopathy.
From the side of the central nervous system: dizziness, asthenia, paresthesia, headache, insomnia, fatigue, anxiety, depression.
Visual disorders: visual impairment; conjunctivitis.
From the cardiovascular system: very rarely - arrhythmia, arterial hypotension.
Respiratory diseases, thoracic and mediastinal disorders: cough, pharyngitis, sinusitis, rhinorrhea, rhinitis.
Diseases of the kidneys and urinary tract: renal failure, increased incidence of infections, nocturia, polyuria, increased urea nitrogen, creatinine.
Diseases of the reproductive system and mammary glands: decreased libido, decreased potency.
From the side of metabolism: glucosuria, sweating, weight loss.
Allergic reactions: most are in the form of dermatological reactions, especially in patients prone to allergies: maculopapular rash, purpura, exacerbation of systemic lupus erythematosus, angioedema and / or urticaria, toxic epidermal necrolysis, Stevens-Johnson syndrome, photosensitivity, hemorrhagic vasculitis.
Hematological disorders: very rarely - thrombocytopenia, leukopenia, agranulocytosis, hemolytic anemia, aplastic anemia.
General diseases and situational conditions for the administration of drugs: flu-like syndrome, pain in the chest, back, infections, exacerbation of systemic lupus erythematosus.
Enalapril. with caution, enalapril should be prescribed in case of bilateral renal artery stenosis, single kidney artery stenosis, hyperkalemia, condition after kidney transplantation, aortic stenosis, mitral stenosis (with hemodynamic impairment), in idiopathic hypertrophic subaortic stenosis, systemic diseases of connective tissue, urosculosis, ulcer disease with diabetes mellitus, liver failure, patients who follow a salt-restricted diet or are on chronic hemodiosis Lease with the simultaneous intake of immunosuppressants and saluretikov, the elderly (65 years).
Patients should be monitored for 2 hours after taking the initial dose of the drug and an additional 1 hour until stabilization of blood pressure.
With caution, appoint persons with impaired renal function. A decrease in the excretion of active ACE inhibitors leads to an increase in their concentration in blood plasma and the risk of hyperkalemia, proteinuria, neutropenia and agranulocytosis. Such patients may require lower doses or less frequent use and a moderate increase in doses.
In the case of previous treatment with saluretics, in particular in patients with chronic heart failure, the risk of developing orthostatic hypotension increases, therefore, before starting treatment, it is necessary to compensate for the loss of fluid and salts.
Before examining the function of the parathyroid glands, enalapril should be discontinued.
In the case of the development of angioedema of the face and neck, the drug should be discontinued and antihistamines prescribed. Severe cases of angioedema of the tongue, glottis and / or larynx require emergency use of epinephrine and support for patency of the upper respiratory tract (intubation, tracheotomy).
During surgical interventions during the treatment with enalapril, the development of arterial hypotension is possible, which should be corrected by the introduction of a sufficient amount of fluid.
It is not recommended to prescribe the drug to patients undergoing hemodialysis using polyacrylonitrile membranes before using dextran preparations or performing specific desensitization to bee and wasp venom, since the use of enalapril can lead to anaphylactic reactions.
Patients taking enalapril should not drink alcohol because of the risk of developing arterial hypotension.
During long-term therapy, control of the peripheral blood picture is shown (hemoglobin concentration, red blood cell count, white blood cell count, platelet count, white blood cell count, ESR determination).
Cough. Cough has been reported during treatment with ACE inhibitors. Usually a cough is unproductive persistent and stops after discontinuation of the drug. Cough due to treatment with an ACE inhibitor must be considered in the differential diagnosis of cough.
With caution, enalapril should be used in the presence of primary hyperaldosteronism.
Surgery / anesthesia. During major surgeries or during anesthesia with drugs that cause arterial hypotension, enalapril blocks the formation of angiotensin II for the second time to compensatory renin release. If arterial hypotension develops, which can be explained by these interaction mechanisms, it is corrected by increasing the volume of fluid.
Potassium in blood plasma. See INTERACTIONS.
Lactose. Since the drug contains lactose, it is contraindicated for use in patients with lactose intolerance.
Indapamide. Precautions are prescribed for diabetes mellitus in the stage of decompensation, hyperuricemia (especially accompanied by gout and urate nephrolithiasis).
Patients taking cardiac glycosides, laxatives against the background of hyperaldosteronism, as well as elderly people, are shown regular monitoring of the content of K ions+ and creatinine.
While taking indapamide, the concentration of K ions should be systematically monitored+Na+Mg2+ in blood plasma (electrolyte disturbances may develop), pH, concentration of glucose, uric acid and residual nitrogen.
The most careful control is shown for patients with cirrhosis of the liver (especially with edema or ascites - the risk of developing metabolic alkalosis, which increases the manifestations of hepatic encephalopathy), coronary heart disease, heart failure, and elderly people. An increased risk group also includes patients with an extended Q – T interval on the ECG (congenital or acquired), who should be prescribed the drug with caution.
The first measurement of concentration K+ in the blood should be carried out during the 1st week of treatment. Hypercalcemia with indapamide may be due to previously undiagnosed hyperparathyroidism.
In patients with diabetes mellitus, it is necessary to control the level of glucose in the blood, especially in the presence of hypokalemia.
Significant dehydration can lead to the development of acute renal failure (decreased glomerular filtration). Patients should be compensated for the loss of water and carefully monitor renal function at the beginning of treatment.
Indapamide can give a positive result when conducting a doping control.
Patients with hypertension and hyponatremia (due to the intake of diuretics) need to stop using diuretics (indalapril) 3 days before the start of the use of ACE inhibitors (indapamide), the administration of indapamide is resumed a little later, or ACE inhibitors are initially prescribed in low doses.
Derivatives of sulfonamides can exacerbate the course of systemic lupus erythematosus (this must be borne in mind when prescribing indapamide).
The simultaneous use of enalapril and indapamide leads to an increase in their antihypertensive effect.
Use during pregnancy or lactation. Enalapril and indapamide are contraindicated during pregnancy or lactation.
Children. The effectiveness and safety of the drug in children has not been studied enough, therefore, the drug should not be prescribed to patients of this age category.
The ability to influence the reaction rate when driving vehicles or working with other mechanisms. If there is a need to drive vehicles or work with various machines, then the occurrence of possible dizziness or the onset of fatigue, which may occur as a result of enalapril, should be considered.
Enalapril. diuretic drugs containing potassium, or drugs that supply potassium. APF inhibitors reduce the loss of potassium induced by diuretic drugs. diuretics that store potassium (such as spironolactone, eplerenone, triamteren, or amiloride), substances that supply potassium, or salt substitutes with potassium can lead to a significant increase in the concentration of potassium in the blood plasma. if the simultaneous use is indicated because of the identified hypokalemia, then they should be used with special precautions and with frequent and constant monitoring of the concentration of potassium in the blood plasma.
Diuretic drugs (thiazide or loop diuretics). Preliminary treatment with high-dose diuretics can result in a loss of fluid volume and the risk of arterial hypotension if enalapril therapy is started. The antihypertensive effect can be weakened by stopping the intake of diuretics, increasing the volume of fluid or taking saline, or due to the initial treatment with enalapril in a low dose.
Other antihypertensive drugs. The simultaneous use of these substances can enhance the hypotensive effect of enalapril. Combined use with nitroglycerin or other nitrates or other vasodilators may further reduce blood pressure.
Lithium. There have been reports of a reversible increase in plasma lithium concentration and toxicity while the use of lithium with ACE inhibitors. The combined use of thiazide diuretics can further increase lithium concentrations and increase the risk of lithium toxicity with ACE inhibitors. The use of enalapril with lithium is not recommended, but if necessary, such a combination should be closely monitored for plasma concentrations of lithium.
Tricyclic antidepressants / antipsychotics / anesthetics / narcotic drugs. The simultaneous use of various painkillers, tricyclic antidepressants and antipsychotic drugs with ACE inhibitors may result in a subsequent decrease in blood pressure.
NSAIDs. NSAIDs may weaken the antihypertensive effect of ACE inhibitors. NSAIDs and ACE inhibitors have an additive effect on increasing the concentration of potassium in blood plasma, which may result in a decrease in renal function. These effects are usually reversible. Very rarely, acute renal impairment can also occur, in particular in patients at risk for impaired renal function, such as older people or those with a dehydrated body.
Gold. Occasionally, there have been reports of nitritoid reactions (symptoms include facial flushing, nausea, vomiting, and hypotension) in individuals who have been treated with suitable injectable gold (sodium aurothiomalate) along with concomitant use of ACE inhibitors, including enalapril.
Sympathomimetics (drugs that stimulate or suppress the sympathetic nervous system). Sympathomimetic or adrenomimetic drugs can weaken the antihypertensive effect of ACE inhibitors.
Antidiabetic agents.The results of epidemiological studies suggest that the simultaneous use of ACE inhibitors and antidiabetic drugs (insulins, oral hypoglycemic agents) can cause the increasing effect of lowering blood glucose concentrations with a risk of hypoglycemia. This phenomenon, obviously, can manifest itself to a greater extent during the first weeks of combined treatment, as well as in individuals with impaired renal function. Hypoglycemia is an unusual effect, rarely seen as a result of metabolic or metabolic disorders.
Ethanol Ethanol enhances the hypotensive effect of ACE inhibitors.
Indapamide
Unwanted combinations.
Combinations requiring caution
Enalapril. limited data are available to evaluate human overdose. the most significant sign of an overdose of enalapril is severe arterial hypotension, which began in some cases 6 hours after taking the tablets and was accompanied by blockade of the renin-angiotensin system and stupor.Symptoms associated with an overdose of APF inhibitors may include circulatory shock, electrolyte imbalance, the occurrence of renal failure, hyperventilation, tachycardia, bradycardia, dizziness, anxiety and fear, and coughing. it was reported that after taking 300 and 440 mg of enalapril, the concentration of enalaprilat in the blood plasma was 100-200 times higher than those that are usually observed after taking therapeutic doses.
In the treatment of overdose, the recommended method is iv administration of 0.9% sodium chloride solution. With arterial hypotension, the patient should be moved to horizontal position