


 Secure and encrypted payment processing
Secure and encrypted payment processing We ship to over 40 countries including the USA, UK, Europe, Australia and Japan
We ship to over 40 countries including the USA, UK, Europe, Australia and Japan Guaranteed refund or reship if you haven't received your order
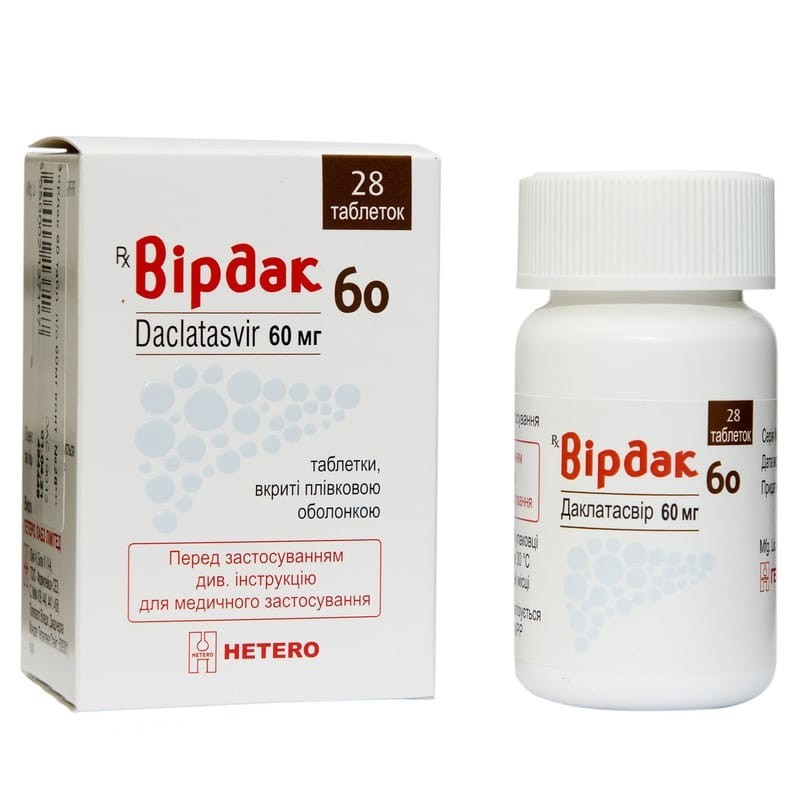
Guaranteed refund or reship if you haven't received your orderFilm-coated tablets Virdac 60 is indicated in combination with other drugs for the treatment of chronic hepatitis C in adults.
Active substance: daclatasvir;
1 tablet contains daclatasvir dihydrochloride, equivalent to 60 mg daclatasvir;
Excipients: lactose, microcrystalline cellulose, croscarmellose sodium, colloidal silicon dioxide, magnesium stearate, Opadry yellow 03B520090 (hypromellose (E 464), titanium dioxide (E 171), macrogol (E 1521), yellow iron oxide (E172).
Hypersensitivity to the active substance or to any of the excipients of the drug.
Simultaneous use with potent inducers of cytochrome P450 3A4 (CYP3A4) and P-gp (e.g. phenytoin, carbamazepine, oxcarbazepine, phenobarbital, rifampicin, rifabutin, rifapentin, systemic dexamethasone and herbal hypericum can reduce expicum per and loss of effectiveness of daclatasvir.
The drug should be used under the supervision of a doctor with experience in the treatment of chronic hepatitis C.
Virdac 60 is used orally regardless of food intake. Patients should be advised that the tablet should be swallowed whole. A film-coated tablet should not be chewed or crushed through the unpleasant taste of the active substance.
Do not use.
Not recommended.
During treatment with daclatasvir in combination with sofosbuvir, dizziness was registered, and during treatment with daclatasvir in combination with interferon alfa and ribavirin, dizziness, impaired attention, blurred vision and decreased visual acuity were recorded.
In clinical studies, there is limited experience with accidental overdose of daclatasvir. In phase 1 clinical trials, healthy volunteers received up to 100 mg 1 time per day for 14 days or single doses up to 200 mg did not have any unpredictable side effects.
There is no known antidote for an overdose of daclatasvir. Treatment of an overdose of daclatasvir should consist of general supportive measures, including monitoring of vital signs, and monitoring the patients clinical condition. Since daclatasvir is strongly bound to proteins (99%) and has a molecular weight of> 500, dialysis is unlikely to significantly reduce the concentration of daclatasvir in blood plasma.
Virdac 60 is contraindicated in combination with strong inducers of CYP3A4 and P-gp (for example, phenytoin, carbamazepine, oxcarbazepine, phenobarbital, rifampicin, rifabutin, rifapentin, systemic dexamethasone and herbal hypericum, which can lead to a decrease in exposure) the effectiveness of the drug Virdac 60.
Store in the original packaging at a temperature not exceeding 30 ° C out of the reach of children.
Shelf life is 2 years.