

 Secure and encrypted payment processing
Secure and encrypted payment processing We ship to over 40 countries including the USA, UK, Europe, Australia and Japan
We ship to over 40 countries including the USA, UK, Europe, Australia and Japan Guaranteed refund or reship if you haven't received your order
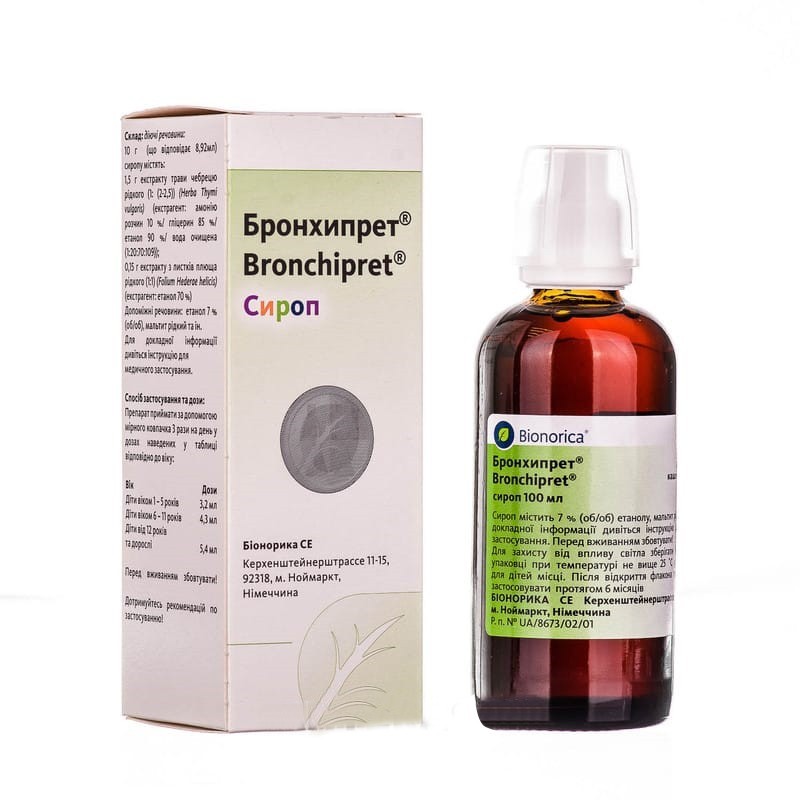
Guaranteed refund or reship if you haven't received your orderBRONCHIPRET® (BRONCHIPRET®)
COMPOSITION AND FORM OF ISSUE:
BRONHIPRET®
cap d / oral. approx. fl. 50 ml
cap d / oral. approx. fl. 100 ml
| Thyme Herb Extract (Herba Thymi) Liquid | 50 g / 100 g |
| Tincture of ivy leaves (Hederae helicis) | 15 g / 100 g |
Other ingredients: citric acid monohydrate, purified water, saccharin sodium dihydrate, ethyl alcohol 19%.
syrup fl. 50 ml
syrup fl. 100 ml
| Thyme Herb Extract (Herba Thymi) Liquid | 15 g / 100 g |
| Ivy Leaf Extract (Hederae helicis) | 1.5 g / 100 g |
Other ingredients: maltitol syrup, potassium sorbate, citric acid monohydrate, purified water.
No. UA / 8673/02/01 from 07/18/2013 to 07/18/2018
BRONHIPRET® TP
tab. post office, No. 20, No. 50, No. 100
| Thyme herb extract (Herba Thymi) dry | 160 mg |
| Primrose Root Extract (Radix Primulae) dry | 60 mg |
Other ingredients: dimethicone, lactose monohydrate, glucose, anhydrous colloidal silicon dioxide, microcrystalline cellulose, magnesium stearate, hypromellose, peppermint flavor, povidone, crospovidone, propylene glycol, sodium saccharin, talc, riboflavin E101, titanium E101, chlorine E101, titanium E101, chlorine E101, titan E101, chlorine E101, titan E101, chlorine E101, chlorine E101, chlorine E101, titan E101, chlorine polyacrylate dispersion.
No. UA / 8674/01/01 from 07/18/2013 to 07/18/2018
PHARMACOLOGICAL PROPERTIES:
the plant components that make up the drug have a secretolytic, bronchodilator and partially antimicrobial effect. Saponins from primrose roots and ivy leaves have a secretolytic effect, while thyme herb essential oils and flavonoids have a bronchodilator and secretolytic effect. The biologically active substances of ivy relax the smooth muscles of the bronchi and prevent the development of bronchospasm. The antibacterial effect of the drug is due to thymol, which is part of thyme essential oil.
Primrose and ivy saponins are slightly resorbed in the intestine. The thymol component of thyme herb essential oil is secreted mainly by the lungs. Thyme herb flavonoids (e.g. apigenin, luteolin) are split by intestinal microflora and are well absorbed. Excreted unchanged or in the form of metabolites, mainly with urine. Thymol is excreted mainly through the lungs.
INDICATIONS:
diseases of the respiratory tract, which are accompanied by coughing and sputum formation, including acute and chronic bronchitis.
APPLICATION:
adults and adolescents over the age of 12 are prescribed 1 tablet before meals 3 times a day. The tablets are swallowed without chewing, washed down with a small amount of liquid.
Drops are prescribed by mouth, adults - 50 drops 3 times a day, adolescents aged 12-18 years - 35 drops 3 times a day, children aged 6-11 years - 30 drops 3 times a day. If necessary, drops are taken with a small amount of liquid.
Shake the syrup before use, take 3 times a day, taking into account the age and body weight of the child:
| Age years | Dosing, ml 3 times a day | Daily dose, ml |
|---|---|---|
| 1—5 | 3,2 | 9,6 |
| 6–11 | 4,3 | 12,9 |
| ≥12 | 5,4 | 16,2 |
If the body weight of a child over the age of 1 year is significantly different from the norm for this age category, the dose is calculated based on the actual body weight: 1 drop per 1 kg of body weight + 10 drops (for example, a dose for a child with a body weight of 20 kg will be: 20 + 10 = 30 drops). It is recommended to drink undiluted syrup with a small amount of liquid (water, juice, tea). For children under the age of 6, the required amount of syrup should be diluted in 1 tablespoon of liquid.
CONTRAINDICATIONS:
hypersensitivity to the components of the drug.
SIDE EFFECTS:
in isolated cases, manifestations of hypersensitivity in the form of a skin rash, gastrointestinal disorders (spasm, nausea, vomiting) are possible; allergic reactions (difficulty breathing, urticaria, swelling of the face, mouth and / or pharynx). If adverse reactions occur, discontinue therapy, consult a doctor.
SPECIAL INSTRUCTIONS:
during pregnancy and lactation, the drug is used only under medical supervision. It can be prescribed to patients with diabetes mellitus, since 1 tablet, a dose of syrup or drops in 1 dose contains less than 0.03 XE.
INTERACTIONS:
Combined administration with antibacterial agents is possible.
OVERDOSE:
may manifest as dyspeptic symptoms, vomiting, and diarrhea. The treatment is symptomatic.
STORAGE CONDITIONS:
in a dry, dark place at temperatures up to 25 ° C. During storage, clouding of the syrup or solution is possible, which does not indicate a change in the activity of the drug. An open bottle with syrup or rum must be used within 6 months.