

 Secure and encrypted payment processing
Secure and encrypted payment processing We ship to over 40 countries including the USA, UK, Europe, Australia and Japan
We ship to over 40 countries including the USA, UK, Europe, Australia and Japan Guaranteed refund or reship if you haven't received your order
Guaranteed refund or reship if you haven't received your orderActive ingredient: bromhexine;
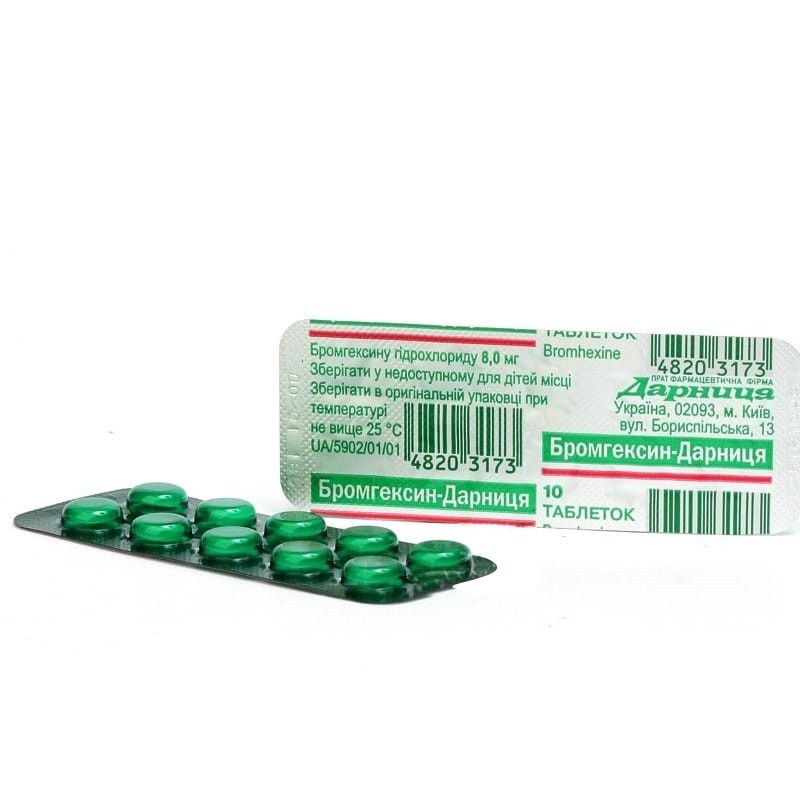
1 tablet contains 8 mg bromhexine hydrochloride;
excipients: lactose monohydrate, potato starch, calcium stearate.
Tablets.
Basic physical and chemical properties: white tablets with a cylindrical shape with a bevel.
Means used for cough and colds. mucolytic agents. code atx r05c b02.
Pharmacodynamics
The drug exhibits a mucolytic (secretolytic) and expectorant effect. The mucolytic effect is associated with depolymerization and rarefaction of mucoprotein and mucopolysaccharide fibers. One of the important features of the action of bromhexine is its ability to stimulate the formation of surfactant - a surfactant of lipid-protein-mucopolysaccharide nature, synthesized in alveoli cells; surfactant biosynthesis is impaired in various bronchopulmonary diseases, which leads to impaired stability of alveolar cells, weakening their response to adverse effects.
Pharmacokinetics
Bromhexine is rapidly absorbed from the gastrointestinal tract. Bioavailability is about 20%. Healthy volunteers have maximum concentration (Cmax) in plasma is determined after 1 hour. The binding of bromhexine to plasma proteins is high. It is widely distributed in body tissues. Bromhexine crosses the blood-brain barrier. In small quantities penetrates the placenta. With prolonged repeated use, it can accumulate. It undergoes intensive metabolism during the first passage through the liver. In the liver, it undergoes demethylation and oxidation. The main metabolite of bromhexine is ambroxol. About 85–90% is excreted in the urine, mainly in the form of metabolites. Half-life (T1/2) is 12–15 hours due to slow back diffusion from the tissues.
Secretolytic therapy for acute and chronic bronchopulmonary diseases, accompanied by complications of sputum discharge.
Hypersensitivity to the components of the drug; peptic ulcer of the stomach and duodenum; diseases of the respiratory system, occurring with the formation of abundant liquid sputum.
Antibiotics (amoxicillin, cefuroxime, erythromycin, doxycycline), sulfonamide drugs - when combined with bromhexine, concentrations of antibiotics and sulfonamides in bronchopulmonary secretion and in sputum increase.
Antitussives - combined use with bromhexine leads to accumulation of mucus in the respiratory tract and difficulty in eliminating sputum from the bronchi against the background of a decrease in cough. This combination is not recommended.
Drugs that irritate the digestive tract (for example, non-steroidal anti-inflammatory drugs) - when combined with bromhexine, mutual enhancement of the effects of mucosal irritation is possible.
Bromhexine-Darnitsa can be prescribed in combination with bronchodilators, antibacterials and with drugs that are used in cardiology.
During treatment, it is necessary to take a sufficient amount of fluid, which enhances the expectorant effect of bromhexine. if there is a history of gastric bleeding, bromhexine should be used under medical supervision.
Use the drug with caution in patients suffering from bronchial asthma and in patients with renal or severe hepatic insufficiency (that is, increase the intervals between doses or reduce the dose).
In acute renal failure, the possibility of accumulation of metabolites of bromhexine in the liver must be considered.
With prolonged use of the drug, it is recommended from time to time to monitor liver function.
The drug contains lactose, so patients with rare hereditary forms of galactose intolerance, lactase deficiency or glucose-galactose malabsorption syndrome cannot use the drug.
At the first manifestations of any violations on the part of the skin or mucous membranes during the use of bromhexine hydrochloride, the use of the drug should be stopped immediately (perhaps this is the beginning of the development of such serious complications as Stevens-Johnson syndrome or Lyell syndrome).
During pregnancy, the drug should be prescribed taking into account the ratio of benefit to the mother / risk to the fetus.
During breastfeeding, the use of bromhexine is contraindicated due to its penetration into breast milk.
There are no reports that the drug may affect the ability to drive a car or work with other mechanisms.
The drug is used orally, regardless of food intake. the duration of admission depends on the indications and course of the disease and is set individually.
Adults and children from 14 years of age: 1-2 tablets (8-16 mg) 3 times a day. The course of treatment is 4–5 days.
Children from 6 to 14 years old, as well as patients with body weight less than 50 kg: 1 tablet (4-8 mg) 3 times a day. The course of treatment is 4–5 days.
In case of impaired renal function or severe liver disease, the dose of the drug must be reduced.
The drug is used for children from 6 years. children under 6 years of age should be used in other dosage forms.
Symptoms: dyspeptic disorders, nausea, vomiting, diarrhea, dizziness, headache, diplopia, ataxia, mild metabolic acidosis, tachypnea. when using up to 40 mg of bromhexine in young children, symptoms were not observed even without decontamination. no chronic toxic effects in humans have been detected.
Treatment: induce vomiting, gastric lavage (in the first 1-2 hours after ingestion), symptomatic therapy. In case of a significant overdose, cardiovascular system should be monitored. Due to the high degree of binding to plasma proteins, the large volume of distribution, and the slow reverse distribution of bromhexine from tissues to the blood, one should not expect accelerated excretion of the drug during hemodialysis or forced diuresis.
From the nervous system: headache (as a migraine), dizziness.
From the respiratory system, chest and mediastinal organs: increased cough, bronchospasm, respiratory distress, respiratory distress.
From the gastrointestinal tract: dyspeptic symptoms, nausea, vomiting, diarrhea, abdominal pain, exacerbation of peptic ulcer of the stomach and duodenum, transient increase in the activity of aminotransferases in blood serum.
On the part of the immune system: hypersensitivity reactions, including skin rashes, erythematic and urticaria rashes, itching of the skin, urticaria; very rarely - Quinckes edema, anaphylactic reactions, including anaphylactic shock; very rarely reported severe skin lesions: Stevens-Johnson syndrome and Lyell syndrome associated with the use of mucolytic agents,such as bromhexine. For the most part, they could be explained by the severity of the underlying disease or the simultaneous administration of another drug. If skin reactions or reactions on the mucous membranes appear, the patient should stop taking the medicine and consult a doctor.
General disorders and changes at the injection site: increased sweating, fever.
3 years.
Store in the original packaging at a temperature not exceeding 25 ° C. Keep out of the reach of children.
On 10 tablets in a blister strip packaging; 5 blisters per pack; 10 or 20 tablets in blisters.
Over the counter.
Ciao “Darnitsa pharmaceutical company”.
Ukraine, 02093, Kiev, st. Borispol, 13.